Merck Serono (Switzerland) and Newron Pharmaceuticals SpA (Italy) have initiated the MOTION (SafinaMide add-On To dopamine agonist for early Idiopathic ParkinsON's disease) study. This study will evaluate the efficacy and safety of two dose regimens of safinamide (50 and 100 mg once daily), as add-on therapy to a stable dose of a single dopamine agonist, compared with dopamine agonist monotherapy. The MOTION study is one of the Phase III trials that constitute the clinical development plan previously discussed with regulatory authorities.
Merck Serono, Newron initiate safinamide study The primary endpoint of the trial is the change in motor symptoms assessed by the change in the Unified Parkinson's Disease Rating Scale1 (UPDRS) Part III score from baseline to week 24. Secondary endpoints include changes in measures of activities of daily living, cognitive functions, global clinical status and health-related quality of life.
Merck Serono has exclusive worldwide rights to develop, manufacture and commercialize safinamide in Parkinson's disease, Alzheimer's disease and other therapeutic applications, as per the agreement signed with Newron in 2006.
HOME
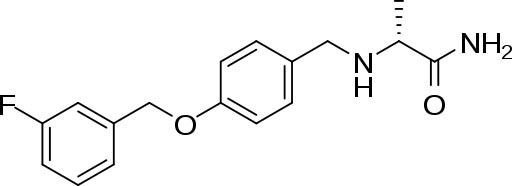
Safinamide
Rasagiline.com
Selegiline.com
Search MEDLINE
Just For Chemists
The Good Drug Guide
The Drug Companies
2010 (Nov) safinamide update
Parkinson's Disease: resources
Depression and Antidepressants
